

It is free of use, saving you the expense of purchasing your own flow analysis software. The workstation is designed to be used for post-acquision analysis. We have a workstation running FlowJo V10 located in SE153. Instrument configuration and fluorochrome guideīD FACS Aria II - Flow cytometer/Cell Sorter Instrument configuration and fluorochrome guideĢ1-color panel example voltages and compensation matrixĤ laser system: 355nm, 405nm, 488nm, 633nmĭesigned for high throughput ananlysis of up to 11 color panelsīD FACS Aria III - Flow cytometer/Cell Sorter with Aerosol ManagementĤ laser system: 405nm, 488nm, 561nm, 633nmĥ61 laser allows maximal detection of dsRed and mCherryĪerosol Management System is required for BSL-2 applications (eg. Below are the SCIF instruments and their specifications: Bio-Rad ZE5 - Flow cytometerĥ laser system: 355nm UV, 405nm Violet, 488nm Blue, 561nm Yellow, 640nm Redĭesigned for high throughput ananlysis of up to 27 color panelsĪutomated tube and plate loading ability up to 384-well plates Please click here to visit our UC Merced-specific FluoroFinder page. We also recommend using FluoroFinder for flow cytometry panel design.

Users can have their samples run with operator assistance, or can run their samples independently with the proper training. ClusterLogic Tutorial 6 Auto-phenotyping. ClusterLogic Tutorial 5 Creating FMO gates used for auto-phenotyping. ClusterLogic Tutorial 4 Exploring clusters. ClusterLogic Tutorial 3 Running the cluster algorithm. ClusterLogic Tutorial 2 Organising your files & defining your parameters.
#Flowjo tutorial video software#
FLOWJO 10 APPLY COMPENSATION MATRIX SOFTWAREįlowJo Basic Tutorial FlowJo is software for the analysis of flow cytometry data.The SCIF is able to assist in design of flow cytometry and FACS experiments. ClusterLogic Tutorial 1 Program settings. This tutorial will quickly introduce you to FlowJo using an example experiment, where a FITC anti-CD8 reagent is titrated to determine the most economical concentration for staining in future experiments This tutorial will introduce you to basic concepts and mechanics of FlowJo vX. The FlowJo Basic Tutorial Video is another way to follow along. Now that you've gotten a handle on how FlowJo works and what types of reports it can generate, try using your data for Special Platforms.

This tutorial is designed to introduce you to the basics of the program. The tutorial data is a 9-color panel designed to interrogate phenotypic and functional responses of human T cell Reading through and performing the steps using the provided data and workspace will give you a feel for the workflow through FlowJo.
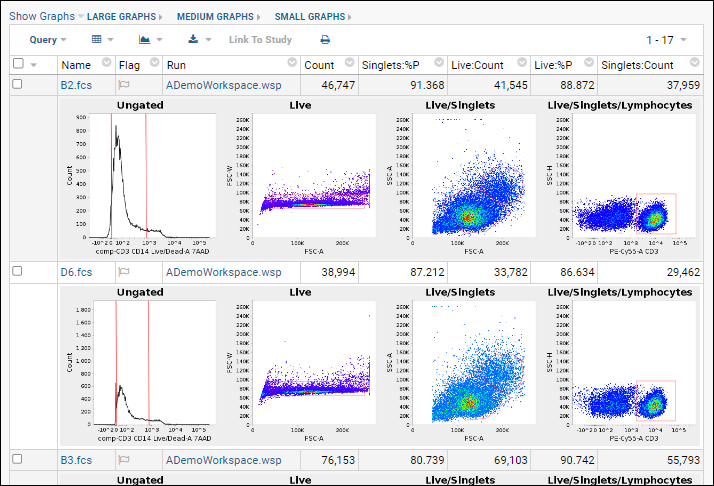
The Plugin Wizard is a useful plugin that can help setup and manage other plugins.
#Flowjo tutorial video how to#
The following video demonstrates how to download and set up an R based plugin for use in FlowJo using the Plugin Wizard. In this FlowJo University tutorial Application Scientist Jack Panopoulos, PhD will walk you through the basics of using the FlowJo layout editor FlowJo™ is the leading analysis platform for single-cell flow and mass cytometry analysis. The following videos explain those new features and lead users through the basic steps necessary to use plugins. Take your data to the next level with the latest tools in FlowJo v10. SeqGeq™ v1.6 analyzes gene expression data-particularly from single-cell RNA sequencing. SeqGeq helps you cluster and subset populations of cells, navigate. This tutorial will quickly introduce you to FlowJo and basic steps for most simple data analysis. FlowJo also provides more advanced applications like compensation and high parameter analysis, as well as special platforms for automated analyses of flow cytometry proliferation experiments with cell-tracking dyes: Overview of tutorials. Additional information about advanced features are.


 0 kommentar(er)
0 kommentar(er)
